Tutorials
TPS is committed to assist organization with Good Manufacturing requirements for medicinal products by implementing the design of Quality system through Providing Policies.
TPS is committed to assist organization with Good Manufacturing requirements for medicinal products by implementing the design of Quality system through Providing Policies.

During the past decades, it has been observed that customers are becoming more and more quality conscious. One of the possible reasons might be that people want to acquire excellence. Pharmaceutical manufacturing industries are facing fierce competition amongst themselves, and to survive, one of the important issues to consider is to establish and implement an appropriate and effective quality system which helps to provide, safer, more efficient and better quality pharmaceutics.
Quality management in pharmaceutical industries, is an important subject because the drugs / or pharmaceutical products are directly delivered to the customer’s body system, thus identity, purity safety and ultimately appropriate quality of product are strongly essential. There are numerous guidelines worldwide that has made some sort of rules and specifications which must be followed by every pharmaceutical industry.

The manufacturing and use of a drug (medicinal) product, including its components, necessarily entail some degree of risk. The risk to its quality is just one component of the overall risk. It is important to understand that product quality should be maintained throughout the product lifecycle such that the attributes that are important to the quality of the drug (medicinal) product remain consistent with those used in the clinical studies.

Poor-quality medicines are not only a health hazard, but a waste of money for both governments and individual consumers, since they may contain toxic substances that have been unintentionally added. For example, in Haiti in 1996, more than 80 children died after receiving a syrup for cough and colds containing glycerol contaminated with diethylene glycol .

As you know about Warning Letters, when FDA finds that a manufacturer has significantly violated FDA regulations FDA notifies the manufacturer. This notifications is often in the form of a Warning Letters and published in public to inform other manufacturers about promotional deliciousness.

The U.S food and drug administration (FDA) inspected drug manufacturing firm, Indoco Remedies Limited and sent a warning letters in Jul. 16. 2019. Here we summarized and bring some parameters of this letter, to learn and use it in our companies.
To show the importance of CAPA handling, in this article TPS wants to exemplify one of the warning letters which is released for a Canadian pharmaceutical company in the last month of 2019.
During the past decades, it has been observed that customers are becoming more and more quality conscious. One of the possible reasons might be that people want to acquire excellence. Pharmaceutical manufacturing industries are facing fierce competition amongst themselves, and to survive, one of the important issues to consider is to establish and implement an appropriate and effective quality system which helps to provide, safer, more efficient and better quality pharmaceutics. In view of this, regulatory agencies across the world have implemented various stringent and strict guidelines to ensure production of pharmaceutical products with assured quality. Despite of industrial practices, these approaches and guidelines are not clear especially for beginners. In this article, we provide a comprehensive overview of Pharmaceutical Quality System (PQS), as proposed by ICH Q10 and defined by different guidelines in which scopes are wider than GMP and includes pharmaceutical development too.
The concept of current pharmaceutical quality management system is based on an internationally harmonized guidance ICH Q10, which describes a model for a pharmaceutical quality system that encourages the use of science- and risk-based approaches and can be implemented throughout the different stages of a product lifecycle. It serves as an effective quality management system for the pharmaceutical industry [5].
ICH Q10 describes that PQS is a management system to direct and control pharmaceutical company with regard to quality. The design, organization and documentation of the pharmaceutical quality system should be well structured and clear to facilitate common understanding and consistent application. The pharmaceutical quality system should include appropriate processes, resources and responsibilities to provide assurance of the quality of outsourced activities and purchased materials [1].
It has been mentioned and noted in “PICS PE 009-14 part I” that the holder of a Manufacturing Authorisation must manufacture medicinal products so as to ensure that they are fit for their intended use, comply with the requirements of the Marketing Authorisation or Clinical Trial Authorisation, as appropriate, and do not place patients at risk due to inadequate safety, quality or efficacy. To achieve this quality objective reliably there must be a comprehensively designed and correctly implemented Pharmaceutical Quality System incorporating Good Manufacturing Practice and Quality Risk Management [2].
WHO scope for a Pharmaceutical Quality System is a wide-ranging concept covering all matters that individually or collectively influence the quality of a product. Within an organization, quality assurance serves as a management tool [3].
FDA guide explains that: every pharmaceutical product has established identity, strength, purity, and other quality characteristics designed to ensure the required levels of safety and effectiveness.
The central goal of a quality system is the consistent production of safe and effective products and ensuring that these activities are sustainable. Quality professionals are aware that good intentions alone will not ensure good products [4].
All parts of the Pharmaceutical Quality System should be adequately resourced with competent personnel, and suitable and sufficient premises, equipment and facilities. The basic concepts of Quality Management, Good Manufacturing Practice (GMP) and Quality Risk Management are inter-related. A Pharmaceutical Quality System should ensure that:
1- Product realisation is achieved by designing, planning, implementing, maintaining and continuously improving a system that allows the consistent delivery of products with appropriate quality attributes
2- Medicinal products are designed and developed in a way that takes account of the requirements of Good Manufacturing Practice [2].

This diagram illustrates the major features of the ICH Q10 Pharmaceutical Quality System (PQS) model. The PQS covers the entire lifecycle of a product including pharmaceutical development, technology transfer, commercial manufacturing, and product discontinuation as illustrated by the upper portion of the diagram. The PQS augments regional GMPs as illustrated in the diagram. The diagram also illustrates that regional GMPs apply to the manufacture of investigational products.
The next horizontal bar illustrates the importance of management responsibilities explained in Section 2 to all stages of the product lifecycle. The following horizontal bar lists the PQS elements which serve as the major pillars under the PQS model. These elements should be applied appropriately and proportionally to each lifecycle stage recognising opportunities to identify areas for continual improvement.
The bottom set of horizontal bars illustrates the enablers: knowledge management and quality risk management, which are applicable throughout the lifecycle stages. These enablers support the PQS goals of achieving product realisation, establishing and maintaining a state of control, and facilitating continual improvement [1].
Continual improvement of the pharmaceutical quality system could achieved with following:
It should be noted that implementing an effective quality system in a manufacturing organization will require a significant investment of time and resources [4]. However, FDA believes the long-term benefits of implementing a quality system will outweigh the costs.
According to FDA guideline, a robust quality model can provide the controls to consistently produce a product of acceptable quality, if properly implemented.
The model must be described according to four major factors:
A robust quality system will promote process consistency by integrating effective knowledge-building mechanisms into daily operational decisions. Both good manufacturing practice and good business practice require a robust quality system. When fully developed and effectively managed, a quality system will lead to consistent, predictable processes that ensure that pharmaceuticals are safe, effective, and available for the consumer [4].
REFERENCES
Quality management in pharmaceutical industries, is an important subject because the drugs / or pharmaceutical products are directly delivered to the customer’s body system, thus identity, purity safety and ultimately appropriate quality of product are strongly essential [3]. There are numerous guidelines worldwide that has made some sort of rules and specifications which must be followed by every pharmaceutical industry. To maintain quality in pharmaceutical products, Quality Management System is followed.
A quality management system (QMS) is a collection of business processes focused on achieving desired quality by posing a regulatory policy and quality objectives in terms of requirements of the customer. The adoption of a QMS should be a strategic decision of an organization. The design and implementation of an organization’s quality management system is influenced by varying needs, particular objectives, the products provided, the processes employed and the size and structure of the organization [1].
“Quality management in the drug industry: philosophy and essential elements” outlines the general concepts of quality assurance as well as the principal components or subsystems of GMP, which are joint responsibilities of top management and of production and quality control management. These include hygiene, validation, self-inspection, personnel, premises, equipment, materials and documentation.
In the drug industry at large, quality management is usually defined as the aspect of management function that determines and implements the “quality policy”, i.e. the overall intention and direction of an organization regarding quality, as formally expressed and authorized by top management. The basic elements of quality management are:
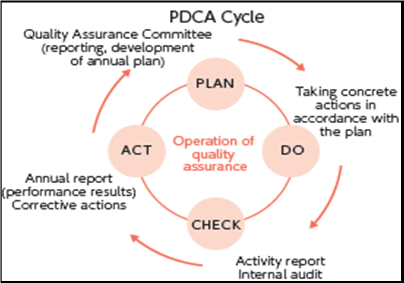
A quality management system typically consists of four facets:
a) Quality planning: Process of translating quality policy into processes, procedures, and instructions to achieve measurable objectives and requirement.
b) Quality assurance: Planned and methodical activities executed as part of a quality system to provide confidence that process, product, or service requirements for quality are being satisfied.
c) Quality control: Act of monitoring, appraising, and correcting a process, product, or service to ensure requirements for quality are being satisfied.
d) Quality improvement: Process of analyzing performance and taking methodical, systemic actions to improve it [3]
The understanding and implementation of appropriate quality management system model enables a pharmaceutical organization to fulfil its ethical as well as regulatory responsibility of including management of identity, quality, safety, purity and efficacy of finished medicinal product. It makes good business sense [4].
REFERENCES:
The manufacturing and use of a drug (medicinal) product, including its components, necessarily entail some degree of risk. The risk to its quality is just one component of the overall risk. It is important to understand that product quality should be maintained throughout the product life cycle such that the attributes that are important to the quality of the drug (medicinal) product remain consistent with those used in the clinical studies. An effective quality risk management approach can further ensure the high quality of the drug (medicinal) product to the patient by providing a proactive means to identify and control potential quality issues during development and manufacturing. Additionally, use of quality risk management can improve the decision making if a quality problem arises. Effective quality risk management can facilitate better and more informed decisions, can provide regulators with greater assurance of a company’s ability to deal with potential risks and can beneficially affect the extent and level of direct regulatory oversight.
Appropriate use of quality risk management can facilitate but does not obviate industry’s obligation to comply with regulatory requirements and does not replace appropriate communications between industry and regulators.
Two primary principles of quality risk management are:
Quality risk management
Quality risk management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across the product life cycle. A model for quality risk management is outlined in the diagram. Other models could be used. The emphasis on each component of the framework might differ from case to case but a robust process will incorporate consideration of all the elements at a level of detail that is commensurate with the specific risk. Overview of a typical quality risk management process is shown in the photo below:

Responsibilities
Quality risk management activities are usually, but not always, undertaken by interdisciplinary teams. When teams are formed, they should include experts from the appropriate areas (e.g. quality unit, business development, engineering, regulatory affairs, production operations, sales and marketing, legal, statistics and clinical) in addition to individuals who are knowledgeable about the quality risk management process.
Initiating a Quality Risk Management Process
Quality risk management should include systematic processes designed to coordinate, facilitate and improve science-based decision making with respect to risk. Possible steps used to initiate and plan a quality risk management process might include the following:
Risk Assessment
Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards (as defined below). Quality risk assessments begin with a well-defined problem description or risk question. When the risk in question is well defined, an appropriate risk management tool and the types of information needed to address the risk question will be more readily identifiable. As an aid to clearly defining the risk(s) for risk assessment purposes, three fundamental questions are often helpful:
Risk identification
Risk identification is a systematic use of information to identify hazards referring to the risk question or problem description
Risk analysis
Risk analysis is the estimation of the risk associated with the identified hazards.
Risk evaluation
Risk evaluation compares the identified and analyzed risk against given risk criteria. Risk evaluations consider the strength of evidence for all three of the fundamental questions.
The output of a risk assessment is either a quantitative estimate of risk or a qualitative description of a range of risk. When risk is expressed quantitatively, a numerical probability is used. Alternatively, risk can be expressed using qualitative descriptors, such as “high”, “medium”, or “low”, which should be defined in as much detail as possible
Risk Control
Risk control includes decision making to reduce and/or accept risks. The purpose of risk control is to reduce the risk to an acceptable level.
Risk reduction
Risk reduction focuses on processes for mitigation or avoidance of quality risk when it exceeds a specified (acceptable) level (see Fig. 1). Risk reduction might include actions taken to mitigate the severity and probability of harm.
Risk acceptance
Risk acceptance is a decision to accept risk. Risk acceptance can be a formal decision to accept the residual risk or it can be a passive decision in which residual risks are not specified.
Risk communication
Risk communication is the sharing of information about risk and risk management between the decision makers and others.
Risk Review
Risk management should be an ongoing part of the quality management process. A mechanism to review or monitor events should be implemented.
The output/results of the risk management process should be reviewed to take into account new knowledge and experience. Once a quality risk management process has been initiated, that process should continue to be utilized for events that might impact the original quality risk management decision, whether these events are planned (e.g. results of product review, inspections, audits, change control) or unplanned (e.g. root cause from failure investigations, recall). The frequency of any review should be based upon the level of risk. Risk review might include reconsideration of risk acceptance decisions.
Additionally, the pharmaceutical industry and regulators can assess and manage risk using recognized risk management tools and/ or internal procedures (e.g., standard operating procedures). Below is a non-exhaustive list of some of these tools:
Quality Risk Management should aim at raising the level of protection for the patient, by the reduction of the risk to which that patient is exposed at the time he receives a drug product [3].
A lot of work has been done on improving the quality of medicinal products and are now being incorporated in the regulatory framework. Still there are a lot of issues in this area which need attention. QRM helps in managing the risks to patients and for the company. Various manufacturing problems still arise at a later period or during batch release, resulting in complicated and costly investigations and other serious quality defects and ultimately results in product recall and in cessation of a batch. The real benefit of applying QRM in medicine manufacturing is to obtain safer medicines for patients. It also allows cost effective and efficient approaches to qualification, validation, change control and other quality control areas [4].
REFERENCES:
Poor-quality medicines are not only a health hazard, but a waste of money for both governments and individual consumers, since they may contain toxic substances that have been unintentionally added. For example, in Haiti in 1996, more than 80 children died after receiving a syrup for cough and colds containing glycerol contaminated with diethylene glycol (1). If the manufacturer had followed good manufacturing practices (GMP), these deaths could have been prevented. In addition, a medicine that contains little or none of the claimed active ingredient will not have the intended therapeutic effect. An antibiotic with some — but not enough — of the active ingredient will not cure infections. Even worse, bacteria exposed to low levels of the antibiotic may not be killed and may become resistant to the drug, even at the correct dosage, putting more lives at risk [1].
WHO defines Good Manufacturing Practices (GMP) as “that part of quality assurance which ensures that products are consistently produced and controlled to the quality standards appropriate to their intended use and as required by the marketing authorization”. GMP covers all aspects of the manufacturing process: defined manufacturing process; validated critical manufacturing steps; suitable premises, storage, transport; qualified and trained production and quality control personnel; adequate laboratory facilities; approved written procedures and instructions; records to show all steps of defined procedures taken; full traceability of a product through batch processing records and distribution records; and systems for recall and investigation of complaints. The guiding principle of GMP is that quality is built into a product, and not just tested into a finished product. Therefore, the assurance is that the product not only meets the final specifications, but that it has been made by the same procedures under the same conditions each and every time it is made. There are many ways this is controlled – controlling the quality of the facility and its systems, controlling the quality of the starting materials, controlling the quality of production at all stages, controlling the quality of the testing of the product, controlling the identity of materials by adequate labelling and segregation, controlling the quality of materials and product by adequate storage, etc. All of these controls must follow prescribed, formal, approved procedures, written as protocols, SOPs, or Master Formulae, describing all the tasks carried out in an entire manufacturing and control process [2].
GMP prevent errors that cannot be eliminated through quality control of the finished product. Without GMP it is impossible to be sure that every unit of medicine is of the same quality as those tested in the laboratory. In the early 1970s, a manufacturer in the United Kingdom produced an infusion fluid which caused the death of five patients because it was heavily contaminated with bacteria (3). Before distributing the fluid, the manufacturer had tested several bottles and found them to be sterile. Eventually a technical fault was found in the sterilizer: the bottles at the bottom had not been properly sterilized. The bottles that the manufacturer had tested were from the upper part, giving the false impression that all the bottles were sterile [1].
Compliance with GMP is a necessary condition for marketing authorization, in other words domestic and foreign producers of pharmaceutical companies cannot sell or market their drugs without it in the foreigner countries. While GMP compliance has not been universally adopted in the developed world, governments in less developed countries are under pressure to comply with GMP requirements when granting marketing authorizations to domestic companies and the other companies has developed a variety of strategies to ensure that developing countries adopt the rules. GMP requirements require major investment in upgrading manufacturing facilities and this has implications for local producers. An interesting empirical question is the impact of these changes on local markets and on access to and affordability of medicines in developing countries. [3]
To obtain and maintain GMP compliance, every manager and supervisor should provide frequent, meaningful GMP reminders, train and develop all employees, and fully participate in formal, ongoing training programs. Senior management must state publicly and make it clear through their actions that following GMPs is the only way their company does business. If you want people to move toward regularly following GMPs, they have to know why: why the regulations came about and what’s in it for all of us as consumers to see them followed. Most requirements were put in place as responses to tragic circumstances and to prevent future tragedies [4].
References:
As you know about Warning Letters, when FDA finds that a manufacturer has significantly violated FDA regulations, FDA notifies the manufacturer. This notifications is often in the form of a Warning Letters and published in public to inform other manufacturers about protentional deliciousness.
From this perspective, our team in TPS attempts to gather the summery of these letters which includes useful and instructive information in order to share them with our most valuable. You can follow this post with the title of Warning Letters & Lessons Learned.
The U.S food and drug administration (FDA) inspected drug manufacturing firm, Indoco Remedies Limited and sent a warning letters in Jul. 16. 2019. Here we summarized and bring some parameters of this letter, to learn and use it in our companies 1.Data integrity remediation The integrity of data is one of the fundamental problems which causes the poor quality system that is not supported the safety and effectiveness of drugs.
2.CGMP Consultant Recommended Based upon the nature of the violation, FDA recommend engaging a consultant highly qualified in data integrity as set forth in 21 CFR 211.34 to assist a firm in meeting CGMP requirements.
3.using of a consultant does not relieve any firm’s obligation to comply with CGMP. the firm’s executive management remains responsible for resolving all deficiencies and systemic flaws to ensure ongoing CGMP compliance.
4.the firm failed to thoroughly investigate any unexplained discrepancy or failure of a batch or any of its components to meet any of its specifications, whether or not the batch has already been distributed.
CAPA plays important role in risk management system. CAPA methodology should result in product and process improvements and enhanced product and process understanding. CAPA is responsible for information collection, analysis, identification and investigation of quality and product problems and taking effective and appropriate preventive and /or corrective actions in an attempt to prevent their occurrence or re-occurrence.
To show the importance of CAPA handling, in this article TPS wants to exemplify one of the warning letters which is released for a Canadian pharmaceutical company in the last month of 2019.
Warning Letter 320-20-15, December 23, 2019:
FDA had an audit on September 2019 and that time the firm failed to routinely calibrate, inspect or check according to a written program designed to assure proper performance and to maintain adequate written records of calibration checks and inspections of automatic, mechanical, electronic equipment, or other types of equipment, including computers, used in the manufacture, processing, packing, and holding of a drug product (21 CFR 211.68(a)).
The company provided CAPA but FDA did not accept their subsequent correspondence, it seems they did not issue sufficient detail or evidence of corrective actions to bring their operations into compliance with CGMP. One example of those gaps in the company’s response is as below:
The company stated that they will randomly select batch records to compare against HMI data and will investigate any discrepancies between batch records and HMI data. FDA declared that response is inadequate due to not commitment to fully evaluate the scope of the discrepancies between HMI data and batch records or practices of operators not following batch instructions. It seems that the company did not establish a root cause for those discrepancies. In addition, they did not expand the investigation to include potential records discrepancies in other operational areas or provide an assessment into distributed product. Furthermore, they did not adequately address the failure of operations management and quality unit (QU) oversight over documentation and data integrity.